Greater sustainability thanks to improved isotope analysis
FAU researchers improve understanding of chemical reactions in water electrolysis
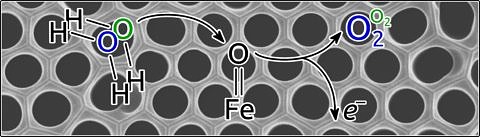
Extracting hydrogen using water electrolysis, that is separating water into oxygen and hydrogen using electric current, could contribute to making energy generated from renewable sources more efficient and cheaper to store in future. This method is still too costly at the moment. An interdisciplinary team of researchers at FAU and the University of Connecticut have now been able to gain a better understanding of chemical reactions during water electrolysis using a new method of analysing oxygen isotopes. The researchers have published their results in the journal Nature Communications.
At first glance, they don’t seem to have much in common. The working group of Prof. Dr. Julien Bachmann researches nanostructured electrode surfaces for increasing the efficiency of energy conversion and storage in fuel cells and electrolysers at the Department of Chemistry and Pharmacy at FAU, while Prof. Dr. Johannes Barth’s working group at the GeoZentrum Nordbayern analyse stable isotopes, cores of elements with varying mass, in the environment. However, for a new interdisciplinary study, the FAU researchers from both working groups have now worked together in order to make water electrolysis more cost effective in future.
Measuring isotopes in their natural composition
The measurement of isotopes is used as a tool in environmental research for determining the origins of bodies of water and the transformation of substances that takes place in these waters. It has also been used for several years to analyse chemical reactions. However, up to now, these measurements have been limited in several ways, especially since monoisotopic substances had to be used. This has now changed due to a high-precision mass spectronomy method for determining isotopes in their natural composition used by the hydrologists at GeoZentrum Nordbayern. ‘This has made the process much easier than previously possible in chemistry’, says Professor Bachmann.
Varying speeds
Sandra Haschke, who is a doctoral candidate in Chemistry at FAU, and her colleagues have now been able to prove that complex reactions in water electrolysis convert the oxygen isotopes of the water at different rates. ‘This depends on the material of the catalysts and the charge applied to the electrode’, explains Bachmann. With the assistance of an expert in this field, Prof. Alfredo Angeles-Boza from the University of Connecticut (USA), who was invited as part of FAU’s visiting professorship programme, new findings have been gained about the individual steps of the reaction on solid surfaces of electrodes. Since such reactions and the surfaces themselves are highly complex and thus difficult to investigate, the new method will provide important information in future for improving electrochemical reactions. ‘If we can improve our understanding of reactions on surfaces, we can further optimise the material for chemical reactions. This means, for example, that it may be possible to replace iridium, which is an excellent but very expensive catalyst, with iron oxide, which is much cheaper, but is currently a very poor catalyst.’
Original publication: http://dx.doi.org/10.1038/s41467-018-07031-1
Further information:
Prof. Dr. Johannes Barth
johannes.barth@fau.de
Prof. Dr. Julien Bachmann
julien.bachmann@fau.de